Background: In MS, the presence of B cells, plasma cells and excess immunoglobulins in central nervous system lesions and in the cerebrospinal fluid implicate the humoral immune system in disease pathogenesis. However, until the advent of specific B-cell-depleting therapies, the critical role of B cells and their products in MS was unproven. Rituximab, a monoclonal antibody that depletes B cells by targeting the CD20 molecule, has been shown to effectively reduce disease activity in MSers with relapsing MS as a single agent. Our investigator-initiated phase II study is the only published clinical trial in which rituximab was used as an add-on therapy in MSers with relapsing MS who had an inadequate response to standard injectable disease-modifying therapies (DMTs).
Methods and results: The primary endpoint, magnetic resonance imaging (MRI) gadolinium-enhanced (GdE) lesion number before versus after rituximab, showed significant benefit of rituximab (74% of post-treatment MRI scans being free of GdE lesions compared with 26% free of GdE lesions at baseline; p < 0.0001). No differences were noted comparing MSers on different DMTs. Several secondary clinical endpoints, safety and laboratory measurements (including B- and T-cell numbers in the blood and cerebrospinal fluid (CSF), serum and CSF chemokine levels, antibodies to myelin proteins) were assessed. Surprisingly, the decline in B-cell number was accompanied by a significant reduction in the number of T cells in both the peripheral blood and CSF. Rituximab therapy was associated with a significant decline of two lymphoid chemokines, CXCL13 and CCL19. No significant changes were observed in serum antibody levels against myelin proteins [myelin basic protein (MBP) and myelin/oligodendrocyte glycoprotein (MOG)] after treatment.
Conclusion: These results suggest that B cells play a role in MS independent from antibody production and possibly related to their role in antigen presentation to T cells or to their chemokine/cytokine production.
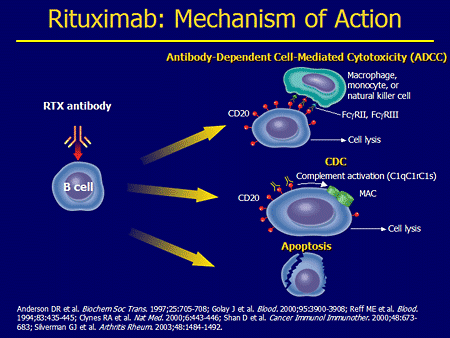
"Anti-B-cell therapies in MS are a potential game-changer! They challenge the T-cell dogma and ask the question of what is causing MS. Do we really knwo what the pathogenesis of the disease is when a B-cell agent is so effective? The bottom line is that most of the DMTs in the high efficacy zone target B cells, i.e. mitoxantrone, alemtuzumab, natalizumab, ocrelizumab, bone marrow transplantation, etc. How are these agents working? One hypothesis I proposed several years ago that has yet to be disproved is that these agents target EBV. Does Rituximab and other anti-B-cell therapies work by reducung EBV loads? You may, or may not, know that Rituximab is the only licensed anti-EBV agent. It is licensed for EBV-associated lymphoproliferative disease. EBV lives in B cells therefore any drug that depletes B cells will have an effect on EBV. We need to do more studies in this area to explore the mechanism of action of these drugs in relation to EBV. Please watch this space as there is a lot of activity in the field."
"Please note this popular cartoon on how rituximab works does not include the EBV hypothesis."
CoI: multiple