Glia. 2014 Jul 8. doi: 10.1002/glia.22719. [Epub ahead of print]
Microglia are rapidly activated in the central nervous system (CNS) in response to a variety of injuries, including inflammation, trauma, and stroke. In addition to modulation of the innate immune response, a key function of microglia is the phagocytosis of dying cells and cellular debris, which can facilitate recovery. Despite emerging evidence that axonal debris can pose a barrier to regeneration of new axons in the CNS, little is known of the cellular and molecular mechanisms that underlie clearance of degenerating CNS axons. We utilize a custom micropatterned microfluidic system that enables robust microglial-axon co-culture to explore the role of Toll-like receptors (TLRs) in microglial phagocytosis of degenerating axons. We find that pharmacologic and genetic disruption of TLR4 blocks induction of the Type-1 interferon response and inhibits phagocytosis of axon debris in vitro. Moreover, TLR4-dependent microglial clearance of unmyelinated axon debris facilitates axon outgrowth. In vivo, microglial phagocytosis of CNS axons undergoing Wallerian degeneration in a dorsal root axotomy model is impaired in adult mice in which TLR4 has been deleted. Since purinergic receptors can influence TLR4-mediated signaling, we also explored a role for the microglia P2 receptors and found that the P2X7R contributes to microglial clearance of degenerating axons. Overall, we identify TLR4 as a key player in axonal debris clearance by microglia, thus creating a more permissive environment for axonal outgrowth. Our findings have significant implications for the development of protective and regenerative strategies for the many inflammatory, traumatic, and neurodegenerative conditions characterized by CNS axon degeneration
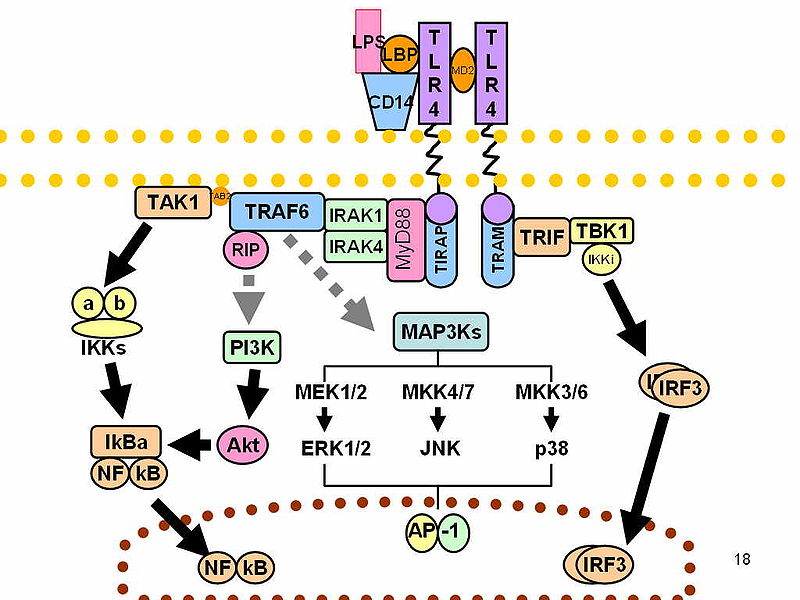
Toll-like receptor 4 is a is a toll-like receptor. It detects lipopolysaccharide from Gram-negative bacteria and is thus important in the activation of the innate immune system. TLR 4 has also been designated as CD284 (cluster of differentiation 284). The protein encoded by this gene is a member of the Toll-like receptor (TLR) family, which plays a fundamental role in pathogen recognition. In this study they report that TLR4 is involved in triggering microglia to remove myelin debris, which allows remyelination to occur. P2X purinoceptor 7 is a protein that in humans is encoded by the P2RX7 gene. The product of this gene belongs to the family of purinoceptors for ATP (cellular energy molecule. The receptor is found in the central and peripheral nervous systems, in microglia, in macrophages, P2X7 receptors have been implicated in ATP-mediated cell death, regulation of receptor trafficking, and inflammation. P2X7 signalling can influence TLR4 which recognise the pathogen-associated molecular patterns (PAMPs) that are expressed by infectious agents. There are many agents that act on P2X7 receptors can they influence myelination in MS?