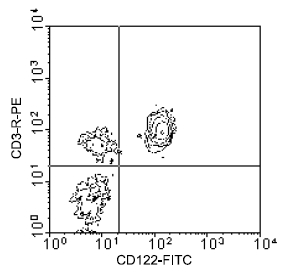
Interleukin-15 (IL-15) is an inflammatory cytokine whose role in autoimmune diseases has not been fully elucidated. Th17 cells have been shown to play critical roles in experimental autoimmune encephalomyelitis (EAE) models. In this study, we demonstrate that blockade of IL-15 signaling by TMβ-1 mAb treatment aggravated EAE severity. The key mechanism was not NK-cell depletion but depletion of CD8+ CD122+ T cells. Adoptive transfer of exogenous CD8+ CD122+ T cells to TMβ-1-treated mice rescued animals from severe disease. Moreover, transfer of pre-activated CD8+CD122+ T cells prevented EAE development and significantly reduced IL-17 secretion. Naïve effector CD4+ CD25- T cells cultured with either CD8+CD122+ T cells from wild-type mice or IL-15 transgenic mice displayed lower frequencies of IL-17A production with lower amounts of IL-17 in the supernatants when compared with production by effector CD4+ CD25- T cells cultured alone. Addition of a neutralizing antibody to IL-10 led to recovery of IL-17A production in Th17 cultures. Furthermore, co-culture of CD8+ CD122+ T cells with effector CD4+ T cells inhibited their proliferation significantly, suggesting a regulatory function for IL-15 dependent CD8+ CD122+ T cells. Taken together, these observations suggest that IL-15, acting through CD8+ CD122+ T cells, has a negative regulatory role in reducing IL-17 production and Th17-mediated EAE inflammation.
Interleukin 15 (IL-15) is a cytokine with structural similarity to IL-2. Like IL-2, IL-15 binds to and signals through a complex composed of IL-2/IL-15 receptor beta chain (CD122) and the common gamma chain (gamma-C, CD132). IL-15 is secreted by mononuclear phagocytes (and some other cells) following infection by virus(es). This cytokine induces cell proliferation of natural killer cells; cells of the innate immune system whose principal role is to kill virally infected cells. interleukin-2 (IL-2) is necessary for the growth, proliferation, and differentiation of thymic-derived lymphocytes (T cells) to become 'effector' T cells. it acts via signalling through CD25. In this study they blocked IL-15 in EAE and things got worse. Not because of effects on natural killer cells but due to depletion of CD8 T cells
Immunology is coming full circle with the return of the CD8 suppressor cell, that express CD122 as previously reported. Thirty-forty years ago CD8 suppressor cells were the mechanism of choice for regulation. Then came along Th2 and T suppressor cells became a dirty word. The findings of yester year may be rediscovered.
However what could it mean for Msers. Well if this subset is useful in humans, then Lemtrada is going to destroy them.It is a sledgehammer to crack a nut and it will destroy useful cells as well as the disease-causing cells. Therefore something that takes out less cells may be better.
Is this yet another part of the zigsaw about why people get secondary autoimmunity after Lemtrada?
In another recent studies these CD8 T cells work by targeting dendritic cells, a type of antigen presenting cell that stimulates T cells.
Kashi VP, Ortega SB, Karandikar NJ. Neuroantigen-Specific Autoregulatory CD8+ T Cells Inhibit Autoimmune Demyelination through Modulation of Dendritic Cell Function. PLoS One. 2014 Aug;9(8):e105763.
Lemtrada depletes dendritic cells too...oops
In another recent studies these CD8 T cells work by targeting dendritic cells, a type of antigen presenting cell that stimulates T cells.
Kashi VP, Ortega SB, Karandikar NJ. Neuroantigen-Specific Autoregulatory CD8+ T Cells Inhibit Autoimmune Demyelination through Modulation of Dendritic Cell Function. PLoS One. 2014 Aug;9(8):e105763.
Lemtrada depletes dendritic cells too...oops