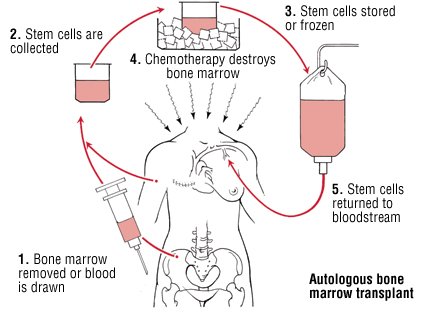
Is hematopoietic stem cell transplant a realistic treatment for MS? #MSBlog #MSResearch
"We are aware that a lot of readers of this blog are proponents of more aggressive therapy. The abstract below is the interim, or 3-year, results of the international autologous hematopoietic stem cell transplant (HCT) trial. The results are very encouraging. Please note that this is an open-label study and hence it is difficult to draw comparisons with other licensed DMTs. NEDA rates at 3-years were a remarkable 78%; higher than any of the licensed DMTs. HCT is not a licensed therapy for MS and hence cannot be offered as part of routine care; at least this is the situation in the UK. How do we get HCT licensed for treating MS? A difficult question; I suspect the community would need to do a head-2-head study against a licensed therapy. I would think the best comparator here would be alemtuzumab considering the potential risk of HCT. It is clear that when HCT is done in specialist units it is much safer than previous studies have suggested."
"These results mirror those of the Canadian BMT collaborative that have been presented at meetings, but have yet to be published in a peer-review journal. On my recent visit to Canada I was told that both Ottawa and Calgary are now providing HCT as a treatment option for MSers who have very active MS and have failed all DMTs. I would be interested to know if their guidelines will include alemtuzumab as it has recently been licensed in Canada."
"Will HCT work for progressive MS? I suspect it will have the same effect as other highly active drugs have in MSers with progressive MS; i.e. it will stop focal MRI activity, prevent any superimposed relapses, but it won't necessarily stop the gradual progression that defines this phase of disease. Unfortunately, we don't have enough long-term data on whether or not there is therapeutic lag with BMT/HSC. However, based on the published trials of BMT in progressive MS most MS centres doing BMT have stopped using BMT/HSC in progressive MS and reserve it for treating early relapsing disease. A situation that is not dissimilar to how we use alemtuzumab."
"What is the difference between autologous bone marrow (BMT) and autologous hematopoietic cell (HCT) transplant? In BMT the stem cells are harvested via a bone marrow aspirate and with HCT they are taken from the peripheral blood after they are mobilised from bone marrow using chemotherapy and in some instances growth factors."
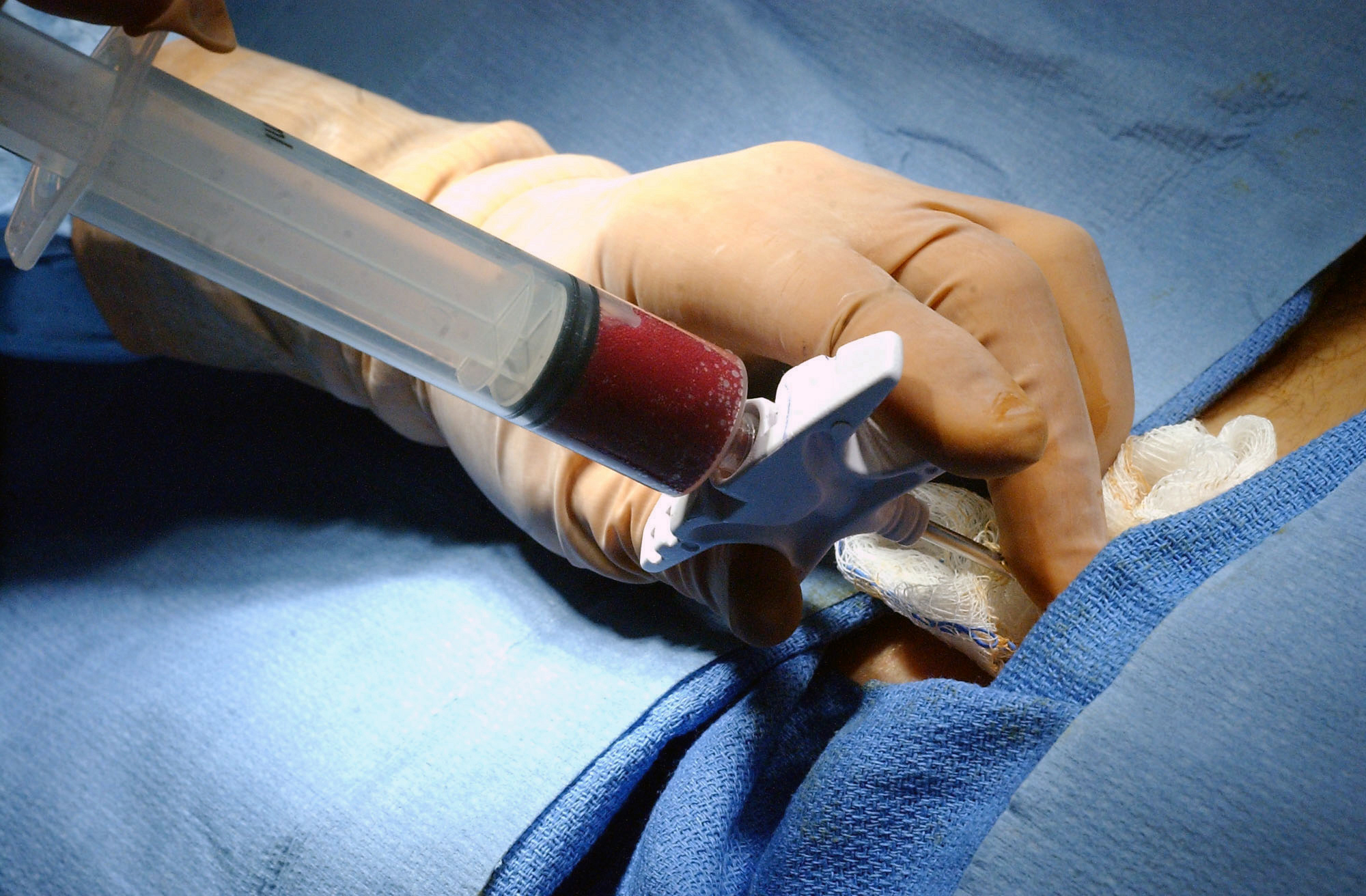 |
| A bone marrow aspirate |
Epub: Nash et al. High-Dose Immunosuppressive Therapy and Autologous Hematopoietic Cell Transplantation for Relapsing-Remitting Multiple Sclerosis (HALT-MS): A 3-Year Interim Report. JAMA Neurol. 2014 Dec 29.
Background: Importance Most patients with relapsing-remitting (RR) multiple sclerosis (MS) who receive approved disease-modifying therapies experience breakthrough disease and accumulate neurologic disability. High-dose immunosuppressive therapy (HDIT) with autologous hematopoietic cell transplant (HCT) may, in contrast, induce sustained remissions in early MS.
Objective: To evaluate the safety, efficacy, and durability of MS disease stabilization through 3 years after HDIT/HCT.
Design, Setting, and Participants: Hematopoietic Cell Transplantation for Relapsing-Remitting Multiple Sclerosis (HALT-MS) is an ongoing, multicenter, single-arm, phase 2 clinical trial of HDIT/HCT for patients with RRMS who experienced relapses with loss of neurologic function while receiving disease-modifying therapies during the 18 months before enrolling. Participants are evaluated through 5 years after HCT. This report is a prespecified, 3-year interim analysis of the trial. Thirty-six patients with RRMS from referral centers were screened; 25 were enrolled.
Interventions: Autologous peripheral blood stem cell grafts were CD34+ selected; the participants then received high-dose treatment with carmustine, etoposide, cytarabine, and melphalan as well as rabbit antithymocyte globulin before autologous HCT.
Main Outcomes and Measures: The primary end point of HALT-MS is event-free survival defined as survival without death or disease activity from any one of the following outcomes: (1) confirmed loss of neurologic function, (2) clinical relapse, or (3) new lesions observed on magnetic resonance imaging. Toxic effects are reported using National Cancer Institute Common Terminology Criteria for Adverse Events.
Results: Grafts were collected from 25 patients, and 24 of these individuals received HDIT/HCT. The median follow-up period was 186 weeks (interquartile range, 176-250) weeks). Overall event-free survival was 78.4% (90% CI, 60.1%-89.0%) at 3 years. Progression-free survival and clinical relapse-free survival were 90.9% (90% CI, 73.7%-97.1%) and 86.3% (90% CI, 68.1%-94.5%), respectively, at 3 years. Adverse events were consistent with expected toxic effects associated with HDIT/HCT, and no acute treatment-related neurologic adverse events were observed. Improvements were noted in neurologic disability, quality-of-life, and functional scores.
Conclusions and Relevance: At 3 years, HDIT/HCT without maintenance therapy was effective for inducing sustained remission of active RRMS and was associated with improvements in neurologic function. Treatment was associated with few serious early complications or unexpected adverse events.
HALT-MS is a prospective, open-label, single-arm, multicenter phase 2 clinical trial (clinicaltrials.gov Identifier: NCT00288626).
Background: Importance Most patients with relapsing-remitting (RR) multiple sclerosis (MS) who receive approved disease-modifying therapies experience breakthrough disease and accumulate neurologic disability. High-dose immunosuppressive therapy (HDIT) with autologous hematopoietic cell transplant (HCT) may, in contrast, induce sustained remissions in early MS.
Objective: To evaluate the safety, efficacy, and durability of MS disease stabilization through 3 years after HDIT/HCT.
Design, Setting, and Participants: Hematopoietic Cell Transplantation for Relapsing-Remitting Multiple Sclerosis (HALT-MS) is an ongoing, multicenter, single-arm, phase 2 clinical trial of HDIT/HCT for patients with RRMS who experienced relapses with loss of neurologic function while receiving disease-modifying therapies during the 18 months before enrolling. Participants are evaluated through 5 years after HCT. This report is a prespecified, 3-year interim analysis of the trial. Thirty-six patients with RRMS from referral centers were screened; 25 were enrolled.
Interventions: Autologous peripheral blood stem cell grafts were CD34+ selected; the participants then received high-dose treatment with carmustine, etoposide, cytarabine, and melphalan as well as rabbit antithymocyte globulin before autologous HCT.
Main Outcomes and Measures: The primary end point of HALT-MS is event-free survival defined as survival without death or disease activity from any one of the following outcomes: (1) confirmed loss of neurologic function, (2) clinical relapse, or (3) new lesions observed on magnetic resonance imaging. Toxic effects are reported using National Cancer Institute Common Terminology Criteria for Adverse Events.
Results: Grafts were collected from 25 patients, and 24 of these individuals received HDIT/HCT. The median follow-up period was 186 weeks (interquartile range, 176-250) weeks). Overall event-free survival was 78.4% (90% CI, 60.1%-89.0%) at 3 years. Progression-free survival and clinical relapse-free survival were 90.9% (90% CI, 73.7%-97.1%) and 86.3% (90% CI, 68.1%-94.5%), respectively, at 3 years. Adverse events were consistent with expected toxic effects associated with HDIT/HCT, and no acute treatment-related neurologic adverse events were observed. Improvements were noted in neurologic disability, quality-of-life, and functional scores.
Conclusions and Relevance: At 3 years, HDIT/HCT without maintenance therapy was effective for inducing sustained remission of active RRMS and was associated with improvements in neurologic function. Treatment was associated with few serious early complications or unexpected adverse events.
HALT-MS is a prospective, open-label, single-arm, multicenter phase 2 clinical trial (clinicaltrials.gov Identifier: NCT00288626).
CoI: multiple; the Royal London Hospital referred patients to the Imperial College Hospital for potential inclusion in this study.