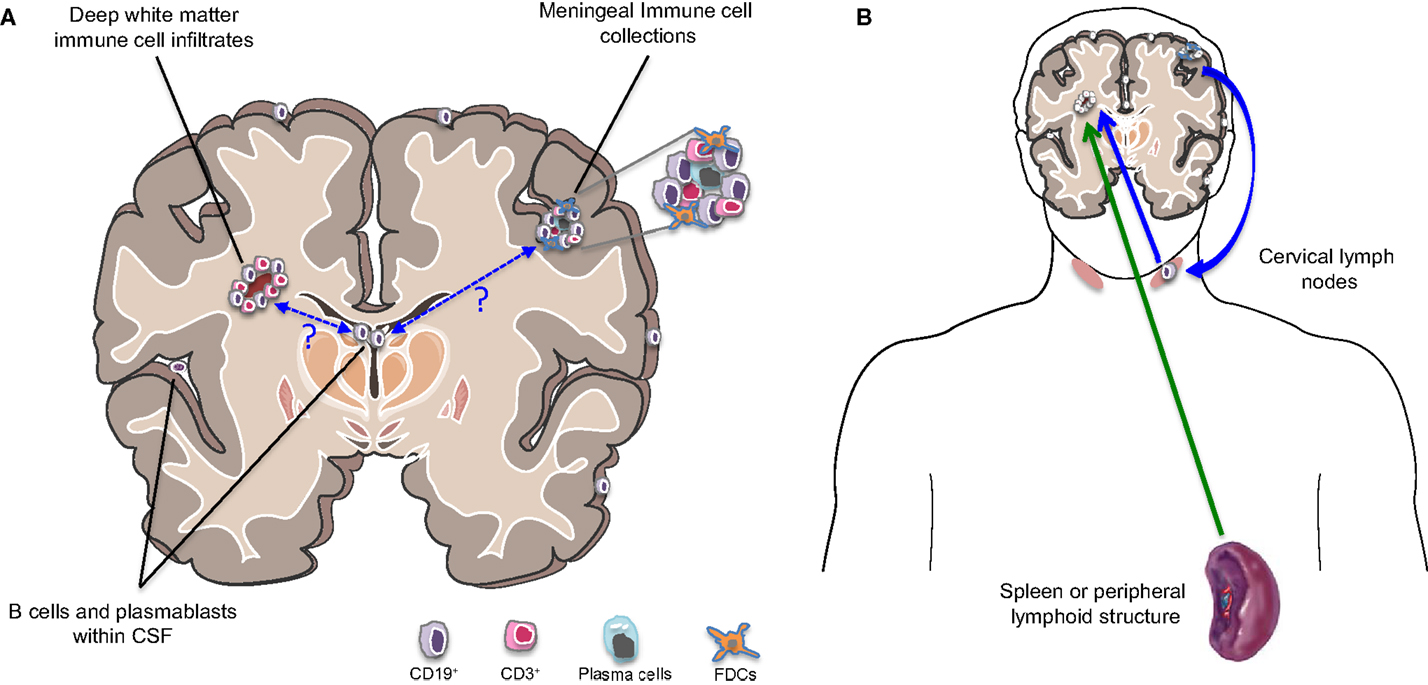
"The trial below which tested the intrathecal at administration of rituximab (anti-CD20) in progressive MS did not have the desired outcome; CSF biomarkers of tissue damage were unchanged. Why? The problem with the lumbar sac, into which the rituximab was injected, is that it is actually a dead end, or cul de sac, and the spinal fluid in this compartment is destined to be flushed out of the CNS into the periphery. Therefore very little rituximab if any would get to the brain to target the B cell follicles. If you want to give intrathecal drugs we really need to give them intraventricularly to allow them to circulate to where we want them to go; i.e. into the sulci and around the veins (Virchow-Robin spaces)."
"In collaboration with a group in St Petersburg, Russia, we showed that administering intrathecal rituximab was similar to giving a dose intravenously; it had a greater impact on peripheral blood B cells that it did on B-cells in the spinal fluid. Based on these findings I wouldn't draw too many conclusions about the lack of efficacy of intrathecal anti-CD20 therapy. It is clear that giving it intravenously is sufficient for efficacy in the relapsing phase of the disease. Whether this strategy will be sufficient in the clinically apparent progressive phase of the disease is another story. I suspect we will need dual therapy, i.e. an anti-CD20 and a neuroprotective. Please note there is very little evidence that anti-CD20 clears the CNS of the plasma cell response and the OCBs (oligoclonal bands). The evidence that the OCBs in MS are part of the pathogenesis of MS and if we really want to make a difference to MS in the long-term we need to clear them for the brain, spinal cord and CSF. For the latter we will need a whole new approach to the treatment of MS. We are however, on the case, we are exploring various different treatment options to target the intrathecal plasma cell response."
OBJECTIVE: Inaccessibility of the inflammation compartmentalized to the central nervous system (CNS) may underlie the lack of efficacy of immunomodulatory treatments in progressive multiple sclerosis (MS). The double blind combination of Rituximab by IntraVenous and IntraThecAl injection versus placebo in patients with Low-Inflammatory SEcondary progressive MS (RIVITALISE; NCT01212094) trial was designed to answer: (1) Whether an induction dose of intravenous and intrathecal rituximab efficiently depletes CNS B cells? and (2) If so, whether this leads to global inhibition of CNS inflammation and slowing of CNS tissue destruction?
METHODS: Patients aged 18-65 years were randomly assigned to rituximab or placebo. Protocol-stipulated interim analysis quantified the efficacy of B-cell depletion.
RESULTS: The efficacy on cerebrospinal fluid (CSF) biomarkers failed to reach criteria for continuation of the trial. B-cell-related CSF biomarkers (sCD21 and B-cell activating factor) changed only in the active-treatment arm. While CSF B cells were killed robustly (median -79.71%, P = 0.0176), B cells in CNS tissue were depleted inadequately (~-10-20%, P < 0.0001). Consequently, the T-cell-specific CSF biomarker sCD27 decreased slightly (-10.97%, P = 0.0005), while axonal damage marker, neurofilament light chain did not change. Insufficient saturation of CD20, lack of lytic complement, and paucity of cytotoxic CD56(dim) NK cells contribute to decreased efficacy of rituximab in the CNS.
INTERPRETATION: Biomarker studies reliably quantified complementary pharmacodynamic effects of rituximab in the CNS, exposed causes for poor efficacy and determined that RIVITALISE trial would be underpowered to measure efficacy on clinical outcomes. Identified mechanisms for poor efficacy are applicable to all CNS-inflammation targeting monoclonal antibodies.
Komori et al. Insufficient disease inhibition by intrathecal rituximab in progressive multiple sclerosis Ann Clin Transl Neurol. 2016 Feb 1;3(3):166-79.
OBJECTIVE: Inaccessibility of the inflammation compartmentalized to the central nervous system (CNS) may underlie the lack of efficacy of immunomodulatory treatments in progressive multiple sclerosis (MS). The double blind combination of Rituximab by IntraVenous and IntraThecAl injection versus placebo in patients with Low-Inflammatory SEcondary progressive MS (RIVITALISE; NCT01212094) trial was designed to answer: (1) Whether an induction dose of intravenous and intrathecal rituximab efficiently depletes CNS B cells? and (2) If so, whether this leads to global inhibition of CNS inflammation and slowing of CNS tissue destruction?
METHODS: Patients aged 18-65 years were randomly assigned to rituximab or placebo. Protocol-stipulated interim analysis quantified the efficacy of B-cell depletion.
RESULTS: The efficacy on cerebrospinal fluid (CSF) biomarkers failed to reach criteria for continuation of the trial. B-cell-related CSF biomarkers (sCD21 and B-cell activating factor) changed only in the active-treatment arm. While CSF B cells were killed robustly (median -79.71%, P = 0.0176), B cells in CNS tissue were depleted inadequately (~-10-20%, P < 0.0001). Consequently, the T-cell-specific CSF biomarker sCD27 decreased slightly (-10.97%, P = 0.0005), while axonal damage marker, neurofilament light chain did not change. Insufficient saturation of CD20, lack of lytic complement, and paucity of cytotoxic CD56(dim) NK cells contribute to decreased efficacy of rituximab in the CNS.
INTERPRETATION: Biomarker studies reliably quantified complementary pharmacodynamic effects of rituximab in the CNS, exposed causes for poor efficacy and determined that RIVITALISE trial would be underpowered to measure efficacy on clinical outcomes. Identified mechanisms for poor efficacy are applicable to all CNS-inflammation targeting monoclonal antibodies.
CoI: multiple