R.I. Spain, K. Powers, C. Murchison, E. Heriza, F.B. Horak, J. Simon, D.N. Bourdette. ECTRIMS2016
Objective: To determine if Lipoic acid (LA) is neuroprotective, reduces disability and improves quality of life, and is safe in a secondary progressive multiple sclerosis (SPMS) population.
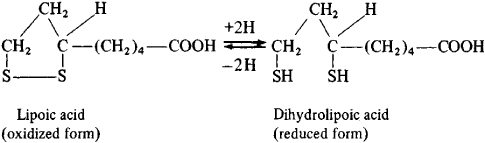
Background: LA is an inexpensive and readily-available oral antioxidant. In the animal model of MS, LA decreases inflammation and reduces optic nerve and spinal cord atrophy. LA reaches therapeutic blood levels and is tolerated at high doses in people with MS. The LA/dihydrolipoic acid redox couple is a key co-factor for pyruvate dehydrogenase in mitochondria which may relate to its mechanism of action in MS.
Methods: A 96 week, double-blind, randomised controlled trial of 1200mg daily LA versus placebo. Primary outcome was reduction in MRI whole brain atrophy. Secondary outcomes included atrophy of brain substructures and spinal cord, retinal and macular atrophy, changes in neurological exam, walking, cognition, fatigue, and quality of life. Adverse events (AEs) and safety laboratory measures were monitored throughout. Intention-to-treat analysis utilised linear mixed-models to evaluate the effect of LA on per 48 week rates of change in outcomes.
Results: Of the 54 randomised participants, 51 (27 LA and 24 placebo) took at least one dose of study drug and were included in analysis. Study arms were overall matched with an average age of 58.5±5.9 (40-69) years, 61 % female, disease duration of 29.6±9.5 (9-51) years, and median EDSS of 6.0 (3.0-9.0). Five participants (9.8 % of total), all taking LA, terminated early. Total and serious AEs were similar between groups, however there was significantly higher gastrointestinal upset and lower numbers of falls in LA participants.
Background: LA is an inexpensive and readily-available oral antioxidant. In the animal model of MS, LA decreases inflammation and reduces optic nerve and spinal cord atrophy. LA reaches therapeutic blood levels and is tolerated at high doses in people with MS. The LA/dihydrolipoic acid redox couple is a key co-factor for pyruvate dehydrogenase in mitochondria which may relate to its mechanism of action in MS.
Methods: A 96 week, double-blind, randomised controlled trial of 1200mg daily LA versus placebo. Primary outcome was reduction in MRI whole brain atrophy. Secondary outcomes included atrophy of brain substructures and spinal cord, retinal and macular atrophy, changes in neurological exam, walking, cognition, fatigue, and quality of life. Adverse events (AEs) and safety laboratory measures were monitored throughout. Intention-to-treat analysis utilised linear mixed-models to evaluate the effect of LA on per 48 week rates of change in outcomes.
Results: Of the 54 randomised participants, 51 (27 LA and 24 placebo) took at least one dose of study drug and were included in analysis. Study arms were overall matched with an average age of 58.5±5.9 (40-69) years, 61 % female, disease duration of 29.6±9.5 (9-51) years, and median EDSS of 6.0 (3.0-9.0). Five participants (9.8 % of total), all taking LA, terminated early. Total and serious AEs were similar between groups, however there was significantly higher gastrointestinal upset and lower numbers of falls in LA participants.
Compliance, measured by pill counts, was >80 %. Annualised rate of whole brain atrophy was significantly lower at 96 weeks in the LA group compared to placebo (slope= -0.22 vs. -0.65, p=0.004). On treatment two year volume loss was -0.4 % (0.7) for LA and (-1.3 % (1.1) for placebo (p=0.006). Walking speed (25 foot walk) nearly improved in the LA group (p=0.057).
Conclusions: This highly promising pilot trial of LA demonstrated a significant reduction in whole brain atrophy and suggested a clinical benefit in SPMS while maintaining safety, tolerability, and high compliance over 96 weeks. A larger trial is necessary to confirm the neuroprotective effects, explore clinical benefits, and ensure the safety of LA in progressive MS populations.
Lipoic acid is an neutriceutical that was reported at ECTRIMS2016. The atrophy rate over the trial (NCT01188811) in the placebo group was 0.65% decrease per year and the lipoic acid group went to 0.22 decrease a year, so not stopping but significant P=0.004 (66%) reduction.
However there was no other outcome that changed like grey matter volume or EDSS (stable or improved in 61% on LA and 71% on placebo, but walking nearly improved.
The main side effects are stomach upsets and stinky pee was mentioned in this stud.y 17% had stomach upsets verses 3 % on placebo. There was kidney failure and protein in urine in two people, but whether this is related required further study.
So this is promising but the question is what next?
We have been here so many times before, the statin trial had promise 6 years ago and still no phase III and surely the neuros attached with this trial need to get the finances to follow this up. But who will fund it?
The NIH have committed to start the process, so one would hope they would continue because, if not surely it is unethical to start. If this is to be worthwhile they will have to do a much larger study likely to involve more than just over 50 and if the FDA want a clinical endpoint which this study was not powered to do, will they get a effect as the EDSS had an increase by 1.33.
There was a suggested effect on walking but this is surely a subset analysis with EDSS up to 9 included. A larger study is needed.
They did not report on the 9 hole peg test so remember #think hand.
However, before we get too excited, I do not believe this is a signal to go down to the health food shop. Time and time again we see small neutriceutical trials going no where. Hopefully this will be different. Will the Progressive Alliance get behind this?
There does appear to be mileage in this. Choi KH, Park M S, Kim JT, Kim HS, Kim JH, Nam TS, Choi SM, Lee SH, Kim BC, Kim MK, Cho KH.Lipoic Acid Use and Functional Outcomes after Thrombolysis in Patients with Acute Ischemic Stroke and Diabetes. PLoS One. 2016;11(9):e0163484
More when it is published.