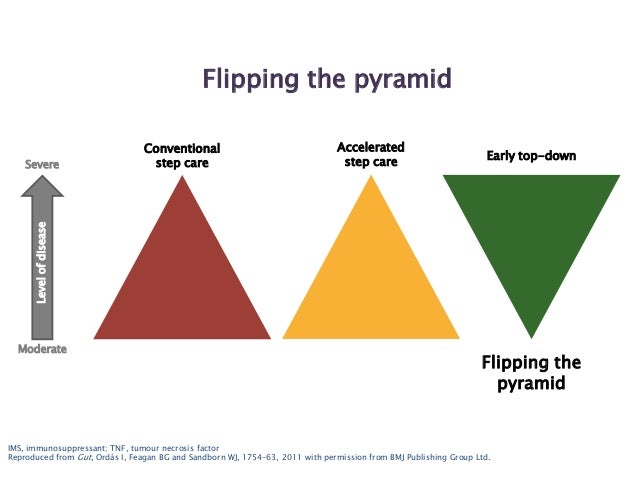
Alemtuzumab 5-year extension data supports flipping the pyramid and hitting MS early and hard. #ResearchSpeak
Summary: The following post summarises the very good 5-year extension data of people with MS treated with alemtuzumab in the pivotal phase 3 trials. In short, the result of very good with most subjects only requiring 2 courses of treatment with a very good outcome. Particularly impressive is the observation of 'normalised' brain volume loss in years 3, 4 and 5.
We have been pushing the treatment paradigm of flipping the pyramid (high-efficacy first-line) for some time now. The CARE-MS 1 study is just this, i.e. these subjects were DMT naive before being treated with alemtuzumab; 68.5% only needed two cycles of treatment in year 1 and 2 with the majority being NEDA in years 3, 4 and 5. The important metric is brain volume loss, a measure of end-organ damage, which was less than or equal to 0.2% per year. This level of brain volume loss is what you see in normal people.
The results in the CARE-MS 2 extension study are no less impressive. These subjects had all failed prior DMTs and hence had a longer disease duration and went onto alemtuzumab with greater underlying damage and/or loss of brain reserve; 60% only needed two cycles of treatment (year 1 and 2), the majority were NEDA and brain volume loss in years 3, 4 and 5 were well under 0.2% per year. Please note brain volume loss in year 1 and 2 are high, this is because of therapeutic lag. Brain volume loss in the next 2 years of your disease is primed by previous inflammatory disease activity hence we would not expect alemtuzumab to impact on this previous damage.
The cons of alemtuzumab are well rehearsed; ~45-50% of subjects will develop secondary autoimmunity over time. The commonest is autoimmune thyroid disease. Alemtuzumab comes with strict monitoring requirements, i.e. monthly blood and urine monitoring for at least 48 months after the last course of treatment. There is also a not so insignificant infection risk in the first few weeks after alemtuzumab infusions. The studies below quote epoch NEDA rates, i.e. NEDA rates per annum. The accumulative rates are lower than this and at 5 years the NEDA rates are less than 50% for both the CARE-MS 1 and 2 studies. The latter is important as this will be the metric we use if and when we do our trial comparing AHSCT to alemtuzumab in the NHS.
Despite these negatives, there is little doubt that alemtuzumab is the most effective DMT we have available to treat people with active MS. Alemtuzumab is also the most cost effective therapy we have licensed to use under the NHS. NHS England would prefer us to use alemtuzumab to all other licensed DMTs because of this. NICE is not the only HTA (health technology appraisal) to come to this conclusion, the Norwegian HTA has drawn the same conclusion. At an NHS service level, alemtuzumab is causing a pharmacovigilance nightmare for us. To see the scale of the problem imagine you treat 100 patient per year with alemtuzumab and you have to do monthly blood and urine monitoring. After 5 years you will have 500 patients requiring monthly monitoring and 500 patients requiring annual MRI scans. This is an industrial-scale logistics problem; it is something we have to gear up for. This is why other emerging DMTs such as oral cladribine and ocrelizumab will have an advantage over alemtuzumab. The monitoring requirements are so much less with these agents that the incentives for MS services to shift to a less arduous mix of DMTs are obvious. For the individual person with MS I would ask your neurologist what the long-term brain atrophy data is for cladribine and ocrelizumab and how they interpret the results. Then you should ask them if they, or one of their close family members, had MS which agent would they choose? I think you may find their answer quite surprising.
Havrdova et al. Alemtuzumab CARE-MS I 5-year follow-up: Durable efficacy in the absence of continuous MS therapy. Neurology. 2017 Aug 23.
OBJECTIVE: To evaluate 5-year efficacy and safety of alemtuzumab in treatment-naive patients with active relapsing-remitting MS (RRMS) (CARE-MS I; NCT00530348).
METHODS: Alemtuzumab-treated patients received treatment courses at baseline and 12 months later; after the core study, they could enter an extension (NCT00930553) with as-needed alemtuzumab retreatment for relapse or MRI activity. Assessments included annualized relapse rate (ARR), 6-month confirmed disability worsening (CDW; ≥1-point Expanded Disability Status Scale [EDSS] score increase [≥1.5 if baseline EDSS = 0]), 6-month confirmed disability improvement (CDI; ≥1-point EDSS decrease [baseline score ≥2.0]), no evidence of disease activity (NEDA), brain volume loss (BVL), and adverse events (AEs).
RESULTS: Most alemtuzumab-treated patients (95.1%) completing CARE-MS I enrolled in the extension; 68.5% received no additional alemtuzumab treatment. ARR remained low in years 3, 4, and 5 (0.19, 0.14, and 0.15). Over years 0-5, 79.7% were free of 6-month CDW; 33.4% achieved 6-month CDI. Most patients (61.7%, 60.2%, and 62.4%) had NEDA in years 3, 4, and 5. Median yearly BVL improved over years 2-4, remaining low in year 5 (years 1-5: -0.59%, -0.25%, -0.19%, -0.15%, and -0.20%). Exposure-adjusted incidence rates of most AEs declined in the extension relative to the core study. Thyroid disorder incidences peaked at year 3 and subsequently declined.
CONCLUSIONS: Based on these data, alemtuzumab provides durable efficacy through 5 years in the absence of continuous treatment, with most patients not receiving additional courses.
Coles et al. Alemtuzumab CARE-MS II 5-year follow-up: Efficacy and safety findings. Neurology. 2017 Aug 23.
OBJECTIVE: To evaluate 5-year efficacy and safety of alemtuzumab in patients with active relapsing-remitting multiple sclerosis and inadequate response to prior therapy.
METHODS: In the 2-year Comparison of Alemtuzumab and Rebif Efficacy in Multiple Sclerosis (CARE-MS) II study (NCT00548405), alemtuzumab-treated patients received 2 courses (baseline and 12 months later). Patients could enter an extension (NCT00930553), with as-needed alemtuzumab retreatment for relapse or MRI activity. Annualized relapse rate (ARR), 6-month confirmed disability worsening (CDW; ≥1-point Expanded Disability Status Scale [EDSS] score increase [≥1.5 if baseline EDSS = 0]), 6-month confirmed disability improvement (CDI; ≥1-point EDSS decrease [baseline score ≥2.0]), no evidence of disease activity (NEDA), brain volume loss (BVL), and adverse events (AEs) were assessed.
RESULTS: Most alemtuzumab-treated patients (92.9%) who completed CARE-MS II entered the extension; 59.8% received no alemtuzumab retreatment. ARR was low in each extension year (years 3-5: 0.22, 0.23, 0.18). Through 5 years, 75.1% of patients were free of 6-month CDW; 42.9% achieved 6-month CDI. In years 3, 4, and 5, proportions with NEDA were 52.9%, 54.2%, and 58.2%, respectively. Median yearly BVL remained low in the extension (years 1-5: -0.48%, -0.22%, -0.10%, -0.19%, -0.07%). AE exposure-adjusted incidence rates in the extension were lower than in the core study. Thyroid disorders peaked at year 3, declining thereafter.
CONCLUSIONS: Alemtuzumab provides durable efficacy through 5 years in patients with an inadequate response to prior therapy in the absence of continuous treatment.
OBJECTIVE: To evaluate 5-year efficacy and safety of alemtuzumab in treatment-naive patients with active relapsing-remitting MS (RRMS) (CARE-MS I; NCT00530348).
METHODS: Alemtuzumab-treated patients received treatment courses at baseline and 12 months later; after the core study, they could enter an extension (NCT00930553) with as-needed alemtuzumab retreatment for relapse or MRI activity. Assessments included annualized relapse rate (ARR), 6-month confirmed disability worsening (CDW; ≥1-point Expanded Disability Status Scale [EDSS] score increase [≥1.5 if baseline EDSS = 0]), 6-month confirmed disability improvement (CDI; ≥1-point EDSS decrease [baseline score ≥2.0]), no evidence of disease activity (NEDA), brain volume loss (BVL), and adverse events (AEs).
RESULTS: Most alemtuzumab-treated patients (95.1%) completing CARE-MS I enrolled in the extension; 68.5% received no additional alemtuzumab treatment. ARR remained low in years 3, 4, and 5 (0.19, 0.14, and 0.15). Over years 0-5, 79.7% were free of 6-month CDW; 33.4% achieved 6-month CDI. Most patients (61.7%, 60.2%, and 62.4%) had NEDA in years 3, 4, and 5. Median yearly BVL improved over years 2-4, remaining low in year 5 (years 1-5: -0.59%, -0.25%, -0.19%, -0.15%, and -0.20%). Exposure-adjusted incidence rates of most AEs declined in the extension relative to the core study. Thyroid disorder incidences peaked at year 3 and subsequently declined.
CONCLUSIONS: Based on these data, alemtuzumab provides durable efficacy through 5 years in the absence of continuous treatment, with most patients not receiving additional courses.
Coles et al. Alemtuzumab CARE-MS II 5-year follow-up: Efficacy and safety findings. Neurology. 2017 Aug 23.
OBJECTIVE: To evaluate 5-year efficacy and safety of alemtuzumab in patients with active relapsing-remitting multiple sclerosis and inadequate response to prior therapy.
METHODS: In the 2-year Comparison of Alemtuzumab and Rebif Efficacy in Multiple Sclerosis (CARE-MS) II study (NCT00548405), alemtuzumab-treated patients received 2 courses (baseline and 12 months later). Patients could enter an extension (NCT00930553), with as-needed alemtuzumab retreatment for relapse or MRI activity. Annualized relapse rate (ARR), 6-month confirmed disability worsening (CDW; ≥1-point Expanded Disability Status Scale [EDSS] score increase [≥1.5 if baseline EDSS = 0]), 6-month confirmed disability improvement (CDI; ≥1-point EDSS decrease [baseline score ≥2.0]), no evidence of disease activity (NEDA), brain volume loss (BVL), and adverse events (AEs) were assessed.
RESULTS: Most alemtuzumab-treated patients (92.9%) who completed CARE-MS II entered the extension; 59.8% received no alemtuzumab retreatment. ARR was low in each extension year (years 3-5: 0.22, 0.23, 0.18). Through 5 years, 75.1% of patients were free of 6-month CDW; 42.9% achieved 6-month CDI. In years 3, 4, and 5, proportions with NEDA were 52.9%, 54.2%, and 58.2%, respectively. Median yearly BVL remained low in the extension (years 1-5: -0.48%, -0.22%, -0.10%, -0.19%, -0.07%). AE exposure-adjusted incidence rates in the extension were lower than in the core study. Thyroid disorders peaked at year 3, declining thereafter.
CONCLUSIONS: Alemtuzumab provides durable efficacy through 5 years in patients with an inadequate response to prior therapy in the absence of continuous treatment.
 |
| Alemtuzumab vs. HSCT: who will come out on top? |
CoI: multiple, I am a co-author on these papers