The "Rumble in le Jungle" at ECTRIMS2017 turned into a "Bumble in the Immunological jungle" as the Heavyweight clash turned into a love-fest.
UPDATE: Slides added
UPDATE: Slides added
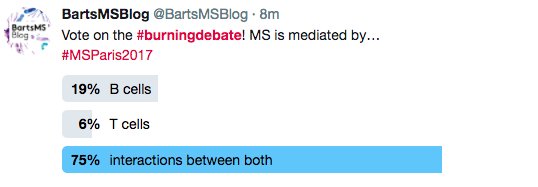
Both contestants had taken too many "peace-pills" rather than defend their corner, both threw in the towel to conclude that T and B cells were both (equally important) central to the problem of MS.
After a promising start, Prof.Hauser slipped on the canvas never recovered and started to verbally-punch himself with counter arguments such that Prof Hafler didn't have to bother defending himself or attacking the alternative view. Just as well, as he forgot his boxing gloves and came with the Immunological Karma Sutra of T and B cell interaction.
With the thought of "Fake News" in both of the American contestants minds, could they defend any extreme position?
Perhaps there was fear of losing?
So much fun was had, that ProfG has gone for Round Two, and this time has persuaded ProfB (Lightweight) to take the challenge to Heinz Weindl (Heavy Weight). Today
But to make it easier for Prof Weindl to accept the challenge, the loop-hole has been closed so that the T and B cells can be the problem as you have to explain how ocrelizumab works, which was the majority view expressed at ECTRIMS, when given the Choice.
So much fun was had, that ProfG has gone for Round Two, and this time has persuaded ProfB (Lightweight) to take the challenge to Heinz Weindl (Heavy Weight). Today
But to make it easier for Prof Weindl to accept the challenge, the loop-hole has been closed so that the T and B cells can be the problem as you have to explain how ocrelizumab works, which was the majority view expressed at ECTRIMS, when given the Choice.
"To B or not-T-B that is the question"; the key pathogenic cell in MS is a B-cell and is independent of antigen presentation to T-cells"
Sounds like ProfB is in for a verbal kicking from the opponent and probably the audience.
However, when the vote went live to twitter sphere after people had watched the videos and could not say both
Rather than a Boxing Match, maybe we should think of if it as a trial with the MS problem in the dock,
There has been a game of Whodunnit called Cludedo?
We have a victim the Ms. Oligodendrocyte has been murd**ed (killed). (If I use the word m******d, it triggers google to make this an adult site).
Where did the event take place?
What was the killing weapon?
We have a victim the Ms. Oligodendrocyte has been murd**ed (killed). (If I use the word m******d, it triggers google to make this an adult site).
Where did the event take place?
We found the Body in the "Brain Room", but is that where the crime was plotted?
What was the killing weapon?
You will hear two theories/cases and the audience can be the Jury to "Acquit One" and "Find the other Guilty" as charged
Can we expect to see the "Chubbacca defence" (deliberately confuse the jury rather than to factually refute the case of the other side) from the Weindl Team
The punishment will be the Death Penalty for the Guilty Party unless there can be rehabilitation.
The debate isn't being filmed, but we will rehearse the arguments from both side after the debate.
*****************************************************
Here are the slides (The Jokes have been removed)
As you can see the task given to ProfB was to argue that B cells can cause MS and this was not mediated by T cells and the activity due to B cells was not antigen presentation.
This view was against what most people thought (about 60%).
(a) The Key Idea presented was that the important subsets would come from looking at the response to therapy.
(b) It was argued by the audience that animal studies show that B cells present antigens to T cells in animals.
It was said because MOG T cell receptor transgenic (developed optic neuritis but not EAE) x MOG B cell receptor (immunoglobulin) transgenic mice (do not get EAE) get EAE means that B cells present antibody to T cells.
This is not necessarily right as MOG T cell receptor transgenic mice, which get optic neuritis in both eyes, get sub-clinical spinal cord EAE. Add anti-MOG antibody and you get augmentation of disease and this can make mice develop clinical EAE, so no B cell presentation occurring.
Although B cells may well present antigens and there is evidence to support, this based on my extreme stance is irrelevant as animal models lack validity, because the CD20 depleting antibodies have a modest/marginal effect compared to that seen in humans. We know animal models are T cell mediated. The aim here was not to discuss any of the animal work. MS is a human disease let's have a human based explanation
(c) Importantly the case was made that T cell depletion had a marginal effect. Now I could argue why the T cell depletion trials had not worked but I was attacking this view,
Again there was uproar by the T cell immunologists when it was suggested by that their much loved Th17 cells didn't do much in MS.
This was contentious as expected. The audience and Prof W argued that IL-17A blockage didn't fail...ProfB's response was he didn't say it failed, he said it was no better than beta interferon, which is hardly a view that interleukin 17 was something magic.
The debate isn't being filmed, but we will rehearse the arguments from both side after the debate.
*****************************************************
Here are the slides (The Jokes have been removed)
At the limits from BartsMSBlog
As you can see the task given to ProfB was to argue that B cells can cause MS and this was not mediated by T cells and the activity due to B cells was not antigen presentation.
This view was against what most people thought (about 60%).
(a) The Key Idea presented was that the important subsets would come from looking at the response to therapy.
(b) It was argued by the audience that animal studies show that B cells present antigens to T cells in animals.
It was said because MOG T cell receptor transgenic (developed optic neuritis but not EAE) x MOG B cell receptor (immunoglobulin) transgenic mice (do not get EAE) get EAE means that B cells present antibody to T cells.
This is not necessarily right as MOG T cell receptor transgenic mice, which get optic neuritis in both eyes, get sub-clinical spinal cord EAE. Add anti-MOG antibody and you get augmentation of disease and this can make mice develop clinical EAE, so no B cell presentation occurring.
Although B cells may well present antigens and there is evidence to support, this based on my extreme stance is irrelevant as animal models lack validity, because the CD20 depleting antibodies have a modest/marginal effect compared to that seen in humans. We know animal models are T cell mediated. The aim here was not to discuss any of the animal work. MS is a human disease let's have a human based explanation
(c) Importantly the case was made that T cell depletion had a marginal effect. Now I could argue why the T cell depletion trials had not worked but I was attacking this view,
Again there was uproar by the T cell immunologists when it was suggested by that their much loved Th17 cells didn't do much in MS.
This was contentious as expected. The audience and Prof W argued that IL-17A blockage didn't fail...ProfB's response was he didn't say it failed, he said it was no better than beta interferon, which is hardly a view that interleukin 17 was something magic.
Activity of secukinumab, an anti-IL-17A antibody, on brain lesions in RRMS: results from a randomized, proof-of-concept study.Havrdová E, Belova A, Goloborodko A, Tisserant A, Wright A, Wallstroem E, Garren H, Maguire RP, Johns DR. J Neurol. 2016;263:1287-95.
The objective of this study was to assess the effect of secukinumab, a monoclonal antibody that inhibits interleukin (IL)-17A, on number of new active brain magnetic resonance imaging (MRI) lesions in subjects with relapsing-remitting multiple sclerosis (MS). Subjects (N = 73) were randomized 1:1 to secukinumab 10 mg/kg or placebo by intravenous infusion at weeks 0, 2, 4, 8, 12, 16, and 20. MRI scans were obtained within 30 days prior to randomization, on a monthly basis during the treatment period, and at study completion. The primary endpoint was the cumulative number of combined unique active lesions (CUAL) observed on brain MRI scans from week 4 to week 24. Compared with placebo, secukinumab non-significantly (MEANS THE TRIAL FAILED as the primary endpoint was not met) reduced the number of CUAL observed on 4-weekly MRI from week 4 to 24 (primary endpoint) by 49 % (95 % CI -10 to 77 %; P = 0.087) and significantly reduced the number of cumulative new gadolinium-enhancing T1 lesions by 67 % (31-84 %, P = 0.003). CUAL reductions were progressively greater from week 4 (1 %) to week 16 (49 %) and persisted until end-study (50 %). There were no serious adverse events; the adverse event rate was comparable to placebo (53 versus 49 %), although mild-to-moderate infection was somewhat more frequent (37 versus 23 %). This proof-of-concept study provides the first evidence that blocking IL-17A with an antibody may reduce MRI lesion activity in MS. Further studies are needed to confirm this finding and determine the magnitude of effect.
The primary endpoint did not reach significance and so the study failed....That's how we do trials.
The primary endpoint did not reach significance and so the study failed....That's how we do trials.
What does Beta interferon do?
Ann Neurol. 1994;36 Suppl:S113-4. This trial was monitored by both clinical and magnetic resonance imaging (MRI) methods. Clinical effect was a 30% reduction in relapse rate in the group treated with a high dose. The MRI activity rate was reduced by a median of 70% in both treatment groups.
De Stefano N, Curtin F, Stubinski B, Blevins G, Drulovic J, Issard D, Shotekov P, Gasperini C; IMPROVE Study Investigators.
Rapid benefits of a new formulation of subcutaneous interferon beta-1a in relapsing-remitting multiple sclerosis. Mult Scler. 2010; 16(7):888-92.
This study evaluated the efficacy of a new formulation of subcutaneous (sc) interferon (IFN)-β1a in relapsing—remitting multiple sclerosis (RRMS). Patients (n = 180) were randomized (2 : 1) to IFN-β1a or placebo for 16 weeks; all patients then received IFN-β1a for 24 weeks. Monthly brain MRI was performed. At week 16, the mean number of combined unique active (CUA) lesions was lower with IFN-β1a than with placebo (p < 0.001; 69% fewer lesions). The mean cumulative number of CUA lesions was already lower with IFN-β1a by week 4 (post hoc analysis; p = 0.015). The new formulation of sc IFN-β1a has rapid beneficial effects on MRI outcomes in RRMS
So anti-IL17 is hardly in the league of something like natalizumab.
Were the T cell immunologists deluding themselves?
(d) An argument was that memory B cells and EBV was interlinked
The question was asked if you have antigen specific B cells, there must be antigen specific T cells. What are they doing?
If the question was turned around and you ask a T celler, what are the antigen specific B cells doing? The answer may be who cares about B cells.
However, if EBV is driving the memory B cell activity,
Does there need to be a T cell help as the virus can make naive B cells proliferate and differentiate into memory B cells and this could be without T cells
There are testable hypothesis that come for these studies,
I suspect the T cellers will continue with their T cell stuff as usual. This is fine meaning we can do it.
However, there was enough open-minded people in the audience to see sense and they are the people who may be treating you.
So anti-IL17 is hardly in the league of something like natalizumab.
Were the T cell immunologists deluding themselves?
However, is it the inhibition of interleukin 17 actually due to Th17 effects? IL-17 is involved in B cell development and can enhance B cell proliferation and germinal center formation. Therefore it could effect memory B cell formation. I guess we will have to wait to see if this is found in psoriasis where the blocking antibody is being used.
(d) The argument was made that Tcellers stop people understanding of how MS works because they view MS as a single cell type. This was clearly evident in the argument of ProfW
(d) The argument was made that Tcellers stop people understanding of how MS works because they view MS as a single cell type. This was clearly evident in the argument of ProfW
(d) An argument was that memory B cells and EBV was interlinked
The question was asked if you have antigen specific B cells, there must be antigen specific T cells. What are they doing?
If the question was turned around and you ask a T celler, what are the antigen specific B cells doing? The answer may be who cares about B cells.
However, if EBV is driving the memory B cell activity,
Does there need to be a T cell help as the virus can make naive B cells proliferate and differentiate into memory B cells and this could be without T cells
There are testable hypothesis that come for these studies,
I suspect the T cellers will continue with their T cell stuff as usual. This is fine meaning we can do it.
However, there was enough open-minded people in the audience to see sense and they are the people who may be treating you.