Yamout BI, El-Ayoubi NK, Nicolas J, El Kouzi Y, Khoury SJ, Zeineddine MM. J. Immunol Res 2018:9084759
OBJECTIVE: To evaluate the efficacy and safety of rituximab in multiple sclerosis in a clinical practice setting.
METHODS:
Clinical data for all adult patients with multiple sclerosis (MS) treated with off-label rituximab at a single MS center in Lebanon between March 2008 and April 2017 were retrospectively collected from medical charts. The main efficacy outcomes assessed were annualized relapse rate (ARR) and proportion of patients free from relapses, disability progression, or magnetic resonance imaging (MRI) activity.
RESULTS:
A total of 89 rituximab-treated patients were included: 59 relapsing-remitting MS (RRMS) and 30 progressive MS (PMS). Patients were treated with 1000 or 2000 mg rituximab IV every 6-12 months for a mean duration of 22.2 ± 24.8 months. The subjects were 65.2% females with a mean age of 40.5 ± 12.3 years and a mean disease duration of 7.9 ± 6.2 years. During treatment, the ARR decreased from 1.07 at baseline to 0.11 in RRMS (p < 0.0001) and from 0.25 to 0.16 in PMS patients (p = 0.593). The mean Expanded Disability Status Scale (EDSS) remained unchanged in both RRMS and PMS patients. Between baseline and the last follow-up, the percent of patients free from any new MRI lesions increased from 18.6% to 92.6% in the RRMS group and from 43.3% to 82% in the PMS group. No evidence of disease activity (NEDA) was achieved in 74% of patients at 1 year of treatment. A total of 64 adverse events (AEs) (71.9%) were recorded with the most common being infusion-related reactions in 25.8% of patients, all mild in nature. Two of our rituximab-treated patients experienced serious AEs requiring surgical interventions: pyoderma gangrenosum vaginalis and an increase in the size of a meningioma. No case of progressive multifocal leukoencephalopathy (PML) was detected.
CONCLUSION:
In our real-world cohort, rituximab was well-tolerated and effective in reducing relapse rate and disability progression in relapsing-remitting and progressive MS patients.
This is real-life use of rituximab, a B cell depleting drug, which is like ocrelizumab, only cheaper...OK it is what we call chimeric, part animal (mouse) and a large part human. Ocelizumab is humanised, a tiny part animal and vast majority human and ofatumumab which is human.
Ocrelizumab binds to CD20 better than rituximab and it is a more effective B cell killer than rituximab.
The main side effect is infusion reactions which was similar to the about 30% infusion reactions in people treated with ocrelizumab.
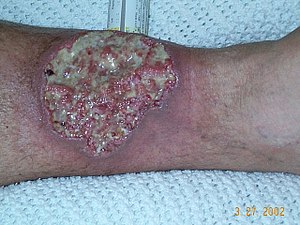
The main adverse events was a meningioma a type of slow growing brain cancer and a form of Pyroderma gangrenosum . This is an unpleasent ulcering condtion thought to a problem with neutrophils, which are the most common type of white blood cell type.
WARNING: Dont view this whilst you are having your breakfast!
It looks unpleasent and it is not even the vaginalis variant.