Larochelle C, Lécuyer MA, Alvarez JI, Charabati M, Saint-Laurent O, Ghannam S, Kebir H, Flanagan K, Yednock T, Duquette P, Arbour N, Prat A.MCAM+ CD8 T lymphocytes mediate CNS inflammation. Ann Neurol. 2015 Apr. doi: 10.1002/ana.24415. [Epub ahead of print]
Asztely F, Gilland E, Wattjes MP, Lycke J.Rituximab treatment did not aggravate ongoing progressive multifocal leukoencephalopathy in a patient with multiple sclerosis. J Neurol Sci. 2015. pii: S0022-510X(15)00207-5. A multiple sclerosis (MS) patient developed progressive multifocal leukoencephalopathy (PML) after 43months of natalizumab treatment. New clinical and magnetic resonance imaging (MRI) findings were initially misinterpreted as breakthrough MS disease activity and natalizumab treatment was replaced by rituximab treatment. The patient had a single infusion of rituximab 1000mg before a definite PML diagnosis was confirmed. Despite undetectable levels of B-cells, JC virus DNA became undetectable in the cerebrospinal fluid by quantitative polymerase chain reaction. The patient partially recovered without any clinical or MRI signs of new MS activity. These findings suggest that B-cell depletion in a non-immune compromised individual did not prevent the patient from clearing the JC virus infection.
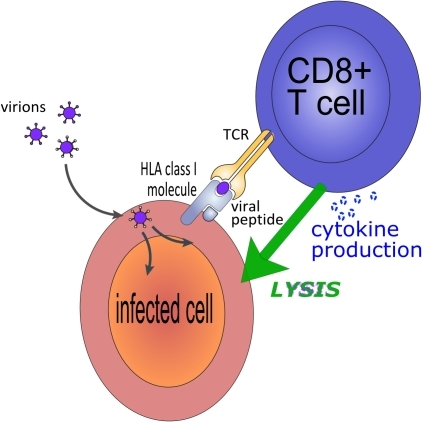
However, remove B cells and T cells then there can be a real problem as we have heard of reports that someone who was switched from Natalizumab on to Alemtuzumab without washout payed the ultimate price because they had PML and therefore no immune system to control this. This points a finger at CD8 cells being important in control of JC virus.
OBJECTIVE: Although Tc17 lymphocytes are enriched in the central nervous system (CNS) of multiple sclerosis (MS) subjects and of experimental autoimmune encephalomyelitis (EAE) animals, limited information is available about their recruitment into the CNS and their role in neuroinflammation. Identification of adhesion molecules used by auto-aggressive CD8+ T lymphocytes to enter the CNS would allow further characterization of this pathogenic subset and could provide new therapeutic targets in MS. We propose that melanoma cell adhesion molecule (MCAM) is a surface marker and adhesion molecule used by pathogenic CD8+ T lymphocytes to access the CNS.
METHODS:Frequency, phenotype and function of MCAM+ CD8+ T lymphocytes was characterized using a combination of ex vivo, in vitro, in situ and in vivo approaches in human and mouse, including healthy controls, MS subjects and EAE animals.
RESULTS: Herein, we report that MCAM is expressed by human effector CD8+ T lymphocytes and it is strikingly up-regulated during MS relapses. We further demonstrate that MCAM+ CD8+ T lymphocytes express more IL-17, IFN-γ, GM-CSF and TNF than MCAMneg lymphocytes, and exhibit an enhanced killing capacity towards oligodendrocytes. MCAM blockade restricts the transmigration of CD8+ T lymphocytes across human blood-brain barrier endothelial cells in vitro, while blocking or depleting MCAM in vivo reduces chronic neurological deficits in active, transfer and spontaneous progressive EAE models.
INTERPRETATION:Our data demonstrate that MCAM identifies encephalitogenic CD8+ T lymphocytes, outlining that MCAM could represent a biomarker of MS disease activity and a valid target for the treatment of neuroinflammatory conditions.
METHODS:Frequency, phenotype and function of MCAM+ CD8+ T lymphocytes was characterized using a combination of ex vivo, in vitro, in situ and in vivo approaches in human and mouse, including healthy controls, MS subjects and EAE animals.
RESULTS: Herein, we report that MCAM is expressed by human effector CD8+ T lymphocytes and it is strikingly up-regulated during MS relapses. We further demonstrate that MCAM+ CD8+ T lymphocytes express more IL-17, IFN-γ, GM-CSF and TNF than MCAMneg lymphocytes, and exhibit an enhanced killing capacity towards oligodendrocytes. MCAM blockade restricts the transmigration of CD8+ T lymphocytes across human blood-brain barrier endothelial cells in vitro, while blocking or depleting MCAM in vivo reduces chronic neurological deficits in active, transfer and spontaneous progressive EAE models.
INTERPRETATION:Our data demonstrate that MCAM identifies encephalitogenic CD8+ T lymphocytes, outlining that MCAM could represent a biomarker of MS disease activity and a valid target for the treatment of neuroinflammatory conditions.
CD146 (cluster of differentiation 146) also known as the melanoma cell adhesion molecule (MCAM) or cell surface glycoprotein MUC18, is a 113kDa cell adhesion molecule MCAM is highly expressed by cells that are components of the blood vessel wall, including vascular endothelial cells, smooth muscle cells and pericytes. Its function is still poorly understood, but evidence points to it being part of the endothelial junction associated with the actin cytoskeleton. Whilst it CD8 are thought to be a problem in MS and blockade of this subset could influence MS, the question may remain whether this would remove the problemof PML. It appears that if you remove B cells then it does not augment the problems of MS
However, remove B cells and T cells then there can be a real problem as we have heard of reports that someone who was switched from Natalizumab on to Alemtuzumab without washout payed the ultimate price because they had PML and therefore no immune system to control this. This points a finger at CD8 cells being important in control of JC virus.
However we have to remember there has been a few PML case after B cell depletion. Is this because the it is the antibody that the Bcleels produce that that fights the infection or are B cells needed for antigen presentation to CD4 cells that help CD8 cells to kill the virus.